Gregor Witte - Hybrid Methods Approaches in DNA Damage
- Research
- Methods
- Scientific Vita
- Publications
- Group Members
Hybrid Method Approaches in DNA-damage recognition, signaling and repair
The maintenance of DNA integrity is an important factor in sustainment of life, and cells monitor this by a variety of DNA damage sensors and checkpoint proteins. While humans have a very complex and mechanistically poorly understood checkpoint control, bacterial systems have a much simpler system that is a promising model system for structural and mechanistic analysis.
We have solved the crystal-structure of a newly identified bacterial DNA-damage sensor protein (DisA-protein; Ben-Yehuda-lab., Cell 2006; 125(4):679-90) that scans the bacterial DNA and binds to DNA recombination intermediate structures (Witte et al., 2008), thereby preventing the cell from entering sporulation phase.
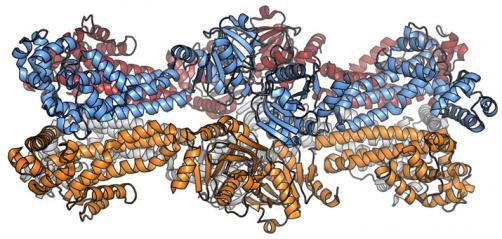
DisA was found to have a previously uncharacterized di-adenylate cyclase enzymatic activity (DAC-activity). The resulting c-di-AMP reaction product is related to the well known c-di-GMP in bacterial physiology. The fact that c-di-AMP has not been described before and no c-di-AMP-regulated proteins are known, opens up a completely new field in bacterial signaling.
C-di-AMP has recently been shown to trigger interferon response of cells upon Listeria infection (Woodward et al., Science 2010;328(5896):1703-5), underlining that it is important also in infection biology and is recognized by the STING protein triggering innate immune response (e.g Burdette et al., Nature 2011;478(7370):515-8).
We aim to analyze the mechanism of DisA and other proteins synthesizing c-di-AMP and identify possible interacting proteins. As a long term goal we aim to identify proteins in the c-di-AMP signaling pathway to get a more complete picture of which processes may be regulated in the cell.

